Category: Uncategorized
-

Quality Management Approach at Pomat Health
At Pomat Health, quality is not just a checkbox; it is the foundation of everything we do. As a full-service Contract Research Organization (CRO), we collaborate with pharmaceutical, biotech, and medical device companies to deliver clinical trials with the highest levels of safety, ethics, and regulatory compliance. From protocol design and trial monitoring to data…
-

Ensuring Participant’s Safety in Clinical Trials
Ensuring Participant Safety in Clinical Trials Introduction The safety of participants in clinical trials is paramount and begins with the meticulous development of the study protocol, the selection of trial sites, and the training and experience of the personnel involved. Clinical trials have significantly advanced medical research, offering new treatments and insights into various health…
-
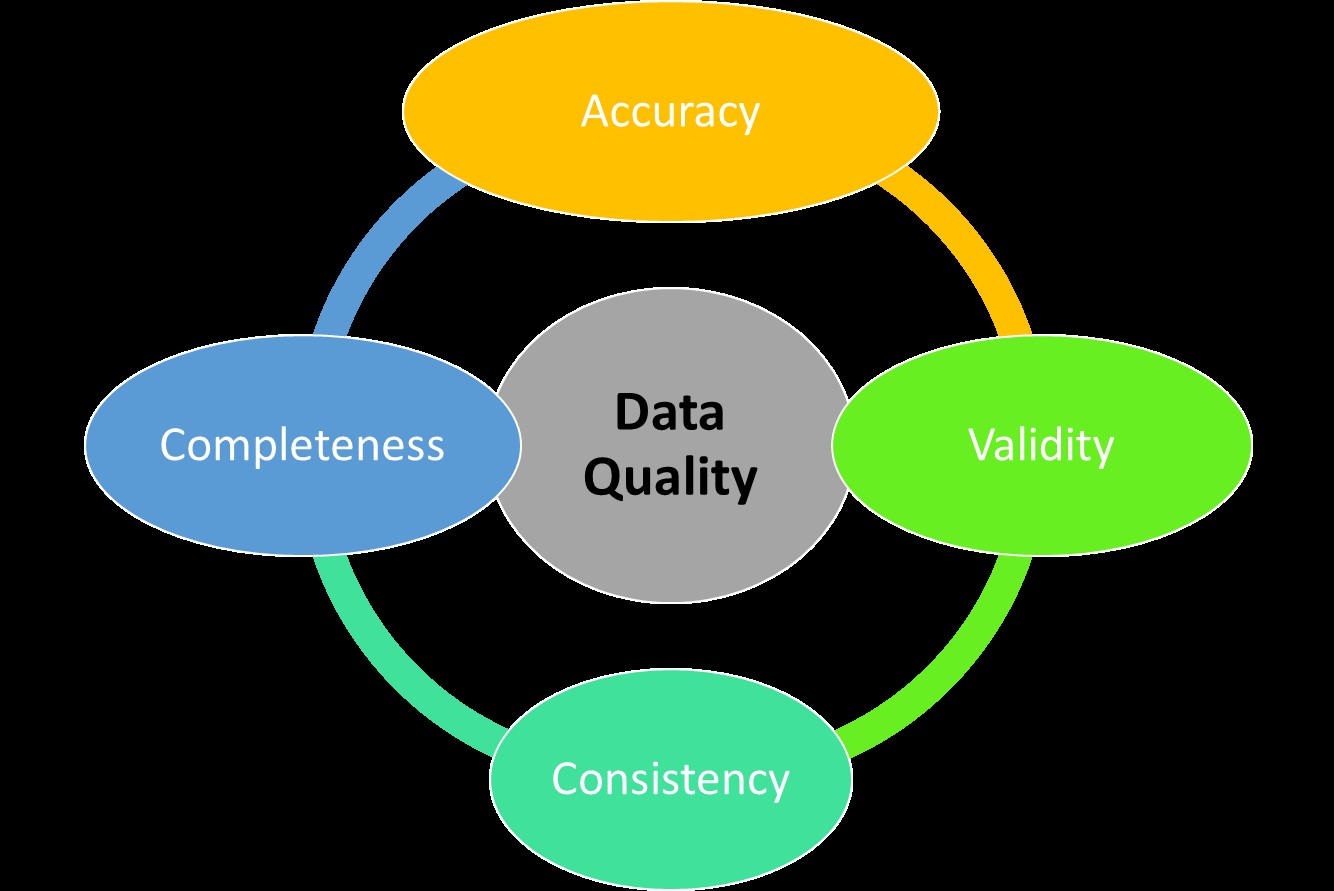
The Importance of Data Quality in Clinical Research
Introduction Data quality in clinical research refers to the reliability and accuracy of qualitative or quantitative information collected during studies. High data quality ensures reliable research findings and avoids incorrect conclusions and recommendations. Errors in data can significantly impact the outcomes of clinical trials, making it essential to prevent data errors during the development, design,…